Descovy
Welcome to Descovy's Media Room
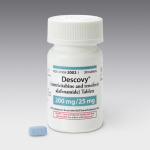
U.S. Food and Drug Administration Approves…
Fixed-Dose Combination HIV Treatment Backbone Can…
Gilead Sciences, Inc. (NASDAQ: GILD) today announced that the U.S. Food and Drug Administration (FDA) has approved Descovy® (emtricitabine 200 mg/tenofovir alafenamide 25 mg, F/TAF), a fixed-dose combination for the treatment of HIV. Descovy is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older.
Open »